-
Notifications
You must be signed in to change notification settings - Fork 6
Home
![]()
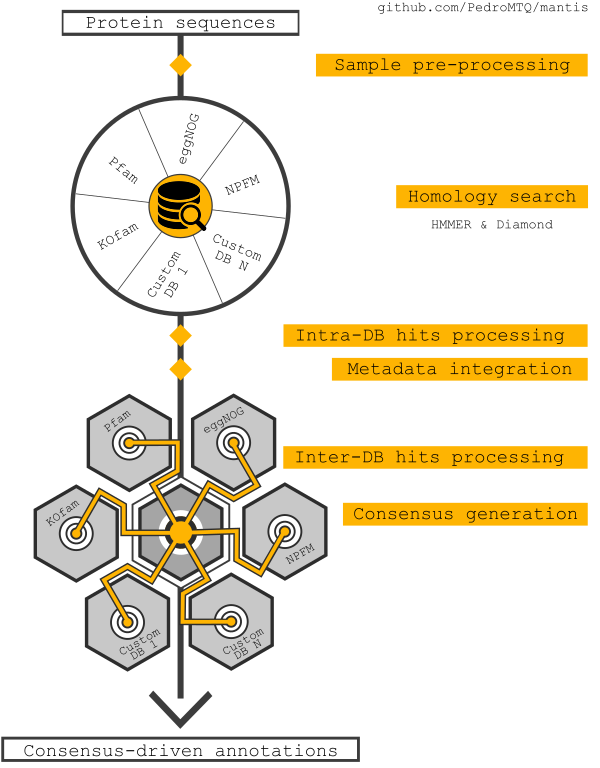
This tool can be used for protein function annotation, it is a standalone tool that uses HMMER or Diamond to match sequences against multiple reference datasets. It accepts as input an aminoacids sequence fasta.
The main goals of this tool are to:
- consider multiple protein domains
- annotate with taxonomy resolution
- use different reference datasets and provide a consensus annotation
- be easy to setup and customize
- scale well with multiple samples and/or metagenomes
If you have only loose reads, you need to assemble them first; when you have assembled reads/genomes you need to predict the protein coding regions (gene prediction - e.g. prodigal) to convert your data into a protein fasta that Mantis can then use.
Mantis is compatible with genomes and metagenomes.
Mantis is compatible with genomes and metagenomes.
| Name | Downloads | Version | Platforms | Latest release |
|---|---|---|---|---|
 |
 |
 |
 |
If you use Mantis, please make sure you cite the respective paper https://doi.org/10.1093/gigascience/giab042
conda install -c bioconda mantis_pfamantis setup
Mantis is now ready to run with: mantis run -i target_faa
Mantis can only run on Linux or MacOS systems. If you want to run Mantis on MacOS make sure you use python 3.7
Custom references can be added in config/MANTIS.cfg by adding their absolute path or folder path, for example:
custom_ref=/path/to/ref_folder/file.hmm
custom_ref=/path/to/ref_folder/file.dmnd
custom_ref=/path/to/ref_folder/
Alternatively you may add them to the custom_refs folder, for example:
Mantis/References/Custom_references/custom1/custom1.hmm
Mantis/References/Custom_references/custom2/custom2.dmnd
You may also redifine the custom_refs folder path by adding your preferred path to custom_refs_folder in the config/MANTIS.cfg file, for example:
custom_refs_folder=path/to/custom_refs/
To integrate metadata, each custom reference folder should contain a metadata.tsv file - see Custom References for more details.
1. Help
mantis -h
2. Setup databases
mantis setup
3. Check installation
mantis check
4. Check SQL metadata file
mantis check_sql
5. Annotate one sample
mantis run -i target.faa -o output_folder-od organism_details -et evalue_threshold -ov overlap_value -mc custom_MANTIS.cfg
example: mantis run -i mantis/tests/test_sample.faa -od "Escherichia coli"
6. Annotate multiple samples
mantis run -i target.tsv -o output_folder -et evalue_threshold -ov overlap_value -mc custom_MANTIS.cfg
example: mantis run -i mantis/tests/test_file.tsv
There are 3 output files:
-
output_annotation.tsv, which has all hits and their coordinates and e-values; -
integrated_annotation.tsvwhich has all hits, their coordinates and e-value, as well as the respective hit metadata; -
consensus_annotation.tsvwhich has all hits and their respective metadata from the best reference sources consensus.
The first two files can have the same query sequence in several lines (query sequence/reference source) while the consensus_annotation.tsv will only have one line per query sequence (consensus/query).
GFF formatted output files can also be generated, as well as KEGG modules completeness tsv. Please see the Output page for information on the additional output files.
This project is available under the MIT license.
Queirós, Pedro, Novikova, Polina, Wilmes, Paul and May, Patrick. "Unification of functional annotation descriptions using text mining" Biological Chemistry, vol. , no. , 2021. https://doi.org/10.1515/hsz-2021-0125
S. R. Eddy. HMMER: biosequence analysis using profile hidden Markov models. HMMER v.3.2.1 www.hmmer.org
Buchfink, B., Xie, C., & Huson, D. H. (2015). Fast and sensitive protein alignment using DIAMOND. Nature methods, 12(1), 59–60. https://doi.org/10.1038/nmeth.3176
eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Jaime Huerta-Cepas, Damian Szklarczyk, Davide Heller, Ana Hernández-Plaza, Sofia K Forslund, Helen Cook, Daniel R Mende, Ivica Letunic, Thomas Rattei, Lars J Jensen, Christian von Mering, Peer Bork Nucleic Acids Res. 2019 Jan 8; 47(Database issue): D309–D314. https://doi.org/10.1093/nar/gky1085
The Pfam protein families database in 2019: S. El-Gebali, J. Mistry, A. Bateman, S.R. Eddy, A. Luciani, S.C. Potter, M. Qureshi, L.J. Richardson, G.A. Salazar, A. Smart, E.L.L. Sonnhammer, L. Hirsh, L. Paladin, D. Piovesan, S.C.E. Tosatto, R.D. Finn Nucleic Acids Research (2019) https://doi.org/10.1093/nar/gky995
Aramaki T., Blanc-Mathieu R., Endo H., Ohkubo K., Kanehisa M., Goto S., Ogata H. KofamKOALA: KEGG ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2019 Nov 19. pii: btz859. https://doi.org/10.1093/bioinformatics/btz859.
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020 Jan 8;48(D1):D265-D268. doi: 10.1093/nar/gkz991. PMID: 31777944; PMCID: PMC6943070.
Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Jaime Huerta-Cepas, Kristoffer Forslund, Luis Pedro Coelho, Damian Szklarczyk, Lars Juhl Jensen, Christian von Mering and Peer Bork. Mol Biol Evol (2017). doi:10.1093/molbev/msx148
Saier MH, Reddy VS, Moreno-Hagelsieb G, Hendargo KJ, Zhang Y, Iddamsetty V, Lam KJK, Tian N, Russum S, Wang J, Medrano-Soto A. The Transporter Classification Database (TCDB): 2021 update. Nucleic Acids Res. 2021 Jan 8;49(D1):D461-D467. doi: 10.1093/nar/gkaa1004. PMID: 33170213; PMCID: PMC7778945.